Calcium metabolism
Reabsorption
[edit]Intestine
[edit]Since about 15 mmol of calcium is excreted into the intestine via the bile per day,[1] the total amount of calcium that reaches the duodenum and jejunum each day is about 40 mmol (25 mmol from the diet plus 15 mmol from the bile), of which, on average, 20 mmol is absorbed (back) into the blood. The net result is that about 5 mmol more calcium is absorbed from the gut than is excreted into it via the bile. If there is no active bone building (as in childhood), or increased need for calcium during pregnancy and lactation, the 5 mmol calcium that is absorbed from the gut makes up for urinary losses that are only partially regulated.[2]
Kidneys
[edit]The kidneys filter 250 mmol of calcium ions a day in pro-urine (or glomerular filtrate), and resorbs 245 mmol, leading to a net average loss in the urine of about 5 mmol/d. The quantity of calcium ions excreted in the urine per day is partially under the influence of the plasma parathyroid hormone (PTH) level - high levels of PTH decreasing the rate of calcium ion excretion, and low levels increasing it.[note 1] However, parathyroid hormone has a greater effect on the quantity of phosphate ions (HPO42−) excreted in the urine.[3] Phosphates form insoluble salts in combination with calcium ions. High concentrations of HPO42− in the plasma, therefore, lower the ionized calcium level in the extra-cellular fluids. Thus, the excretion of more phosphate than calcium ions in the urine raises the plasma ionized calcium level, even though the total calcium concentration might be lowered.
The kidney influences the plasma ionized calcium concentration in yet another manner. It processes vitamin D3 into calcitriol, the active form that is most effective in promoting the intestinal absorption of calcium. This conversion of vitamin D3 into calcitriol, is also promoted by high plasma parathyroid hormone levels.[4][5]
Excretion
[edit]Intestine
[edit]Most excretion of excess calcium is via the bile and feces, because the plasma calcitriol levels (which ultimately depend on the plasma calcium levels) regulate how much of the biliary calcium is reabsorbed from the intestinal contents.
Kidneys
[edit]Urinary excretion of calcium is normally about 5 mmol (200 mg) /day. This is less in comparison to what is excreted via the feces (15 mmol/day).
Regulation
[edit]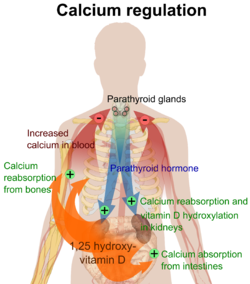
The plasma ionized calcium concentration is regulated within narrow limits (1.3–1.5 mmol/L). This is achieved by both the parafollicular cells of the thyroid gland, and the parathyroid glands constantly sensing (i.e. measuring) the concentration of calcium ions in the blood flowing through them.
High plasma level
[edit]When the concentration of calcium rises, the parafollicular cells of the thyroid gland increase their secretion of calcitonin, a polypeptide hormone, into the blood. At the same time, the parathyroid glands reduce the secretion of parathyroid hormone (PTH), also a polypeptide hormone, into the blood. The resulting high levels of calcitonin in the blood stimulate osteoblasts in bone to remove calcium from blood plasma and deposit it as bone.
The reduced levels of PTH inhibit removal of calcium from the skeleton. The low levels of PTH have several other effects: there is increased loss of calcium in the urine, but more importantly, the loss of phosphate ions through urine is inhibited. Phosphate ions will therefore be retained in the plasma where they form insoluble salts with calcium ions, thereby removing them from the ionized calcium pool in the blood. The low levels of PTH also inhibit the formation of calcitriol (not to be confused with calcitonin) from cholecalciferol (vitamin D3) by the kidneys.
The reduction in the blood calcitriol concentration acts (comparatively slowly) on the epithelial cells (enterocytes) of the duodenum, inhibiting their ability to absorb calcium from the intestinal contents.[7][8][9][10] The low calcitriol levels also act on bone causing the osteoclasts to release fewer calcium ions into the blood plasma.[3]

Low plasma level
[edit]When the plasma ionized calcium level is low or falls the opposite happens. Calcitonin secretion is inhibited and PTH secretion is stimulated, resulting in calcium being removed from bone to rapidly correct the plasma calcium level. The high plasma PTH levels inhibit calcium loss via the urine while stimulating the excretion of phosphate ions via that route. They also stimulate the kidneys to manufacture calcitriol (a steroid hormone), which enhances the ability of the cells lining the gut to absorb calcium from the intestinal contents into the blood, by stimulating the production of calbindin in these cells. The PTH stimulated production of calcitriol also causes calcium to be released from bone into the blood, by the release of RANKL (a cytokine, or local hormone) from the osteoblasts which increases the bone resorptive activity by the osteoclasts. These are, however, relatively slow processes[7][8][3][9][10]
Thus fast short term regulation of the plasma ionized calcium level primarily involves rapid movements of calcium into or out of the skeleton. Long term regulation is achieved by regulating the amount of calcium absorbed from the gut or lost via the feces.[7][8][9][10]
Disorders
[edit]Hypocalcemia (low blood calcium) and hypercalcemia (high blood calcium) are both serious medical disorders. Osteoporosis, osteomalacia and rickets are bone disorders linked to calcium metabolism disorders and effects of vitamin D. Renal osteodystrophy is a consequence of chronic kidney failure related to the calcium metabolism.
A diet adequately rich in calcium may reduce calcium loss from bone with advancing (post-menopausal) age.[11] A low dietary calcium intake may be a risk factor in the development of osteoporosis in later life; and a diet with sustained adequate amounts of calcium may reduce the risk of osteoporosis.
Research
[edit]The role that calcium might have in reducing the rates of colorectal cancer has been the subject of many studies. However, given its modest efficacy, there is no current medical recommendation to use calcium for cancer reduction.
See also
[edit]Footnotes
[edit]- ^ The main determinant of the amount of calcium excreted into the urine per day is the plasma ionized calcium concentration. The plasma parathyroid hormone (PTH) concentration only increases or decreases the amount of calcium excreted at any given plasma ionized calcium concentration. Thus, in primary hyperparathyroidism the quantity of calcium excreted in the urine per day is increased despite the high levels of PTH in the blood. This is because hyperparathyroidism results in hypercalcemia, which increases the urinary calcium concentration (hypercalcuria) despite the modestly increased rate of calcium re-absorption from the renal tubules caused by PTH's effect on those tubules. Kidney stones are therefore often a first indication of hyperparathyroidism, especially since the hypercalcuria is accompanied by an increase in urinary phosphate excretion (a direct result of the high plasma PTH levels). Together the calcium and phosphate tend to precipitate out as water-insoluble salts, which readily form solid “stones”.
References
[edit]- ^ Diem K, Lenter C. Scientific Tables. Vol. 565 (Seventh ed.). Basel: Ciba-Geigy Limited. pp. 653–654. ISBN 978-3-9801244-0-9.
- ^ Barrett KE, Barman SM, Boitano S, Brooks H, "Chapter 23. Hormonal Control of Calcium & Phosphate Metabolism & the Physiology of Bone" (Chapter). Barrett KE, Barman SM, Boitano S, Brooks H: Ganong's Review of Medical Physiology, 23e: http://www.accessmedicine.com/content.aspx?aID=5244785 Archived 2011-07-07 at the Wayback Machine.
- ^ a b c Blaine J, Chonchol M, Levi M (2015). "Renal control of calcium, phosphate, and magnesium homeostasis". Clinical Journal of the American Society of Nephrology. 10 (7): 1257–72. doi:10.2215/CJN.09750913. PMC 4491294. PMID 25287933.
- ^ Stryer L. Biochemistry (Fourth Edition). Chapter 27 "Vitamin D is derived from cholesterol by the ring-splitting action of light". New York, W.H. Freeman and Company.
- ^ Tortora GJ, Anagnostakos NP. Principles of Anatomy and Physiology (Fifth Edition) p. 696. New York, Harper & Row Publishers.
- ^ Boron, Walter F., Boulpaep, Emile L (2003). "The Parathyroid Glands and Vitamin D". Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1094. ISBN 978-1-4160-2328-9.
- ^ a b c Brini M, Ottolini D, Calì T, Carafoli E (2013). "Chapter 4. Calcium in Health and Disease". In Sigel A, Helmut RK (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 81–137. doi:10.1007/978-94-007-7500-8_4. ISBN 978-94-007-7499-5. PMID 24470090.
- ^ a b c Marshall WJ (1995). Clinical Chemistry (3rd ed.). London: Mosby. ISBN 978-0-7234-2190-0.
- ^ a b c Walter F. (2003). "The Parathyroid Glands and Vitamin D in". Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1094. ISBN 978-1-4160-2328-9.
- ^ a b c Guyton A (1976). ‘’Medical Physiology’’. p.1062; New York, Saunders and Co.
- ^ Heaney RP (Apr 2000). "Calcium, dairy products and osteoporosis". Journal of the American College of Nutrition. 19 (2 Suppl): 83S–99S. doi:10.1080/07315724.2000.10718088. PMID 10759135. S2CID 18794160. Archived from the original on 2012-08-03.
External links
[edit]- Calcium at Lab Tests Online
- Nosek TM. "Section 5/5ch6/5ch6line". Essentials of Human Physiology.[dead link]
